Search
-
Pool Safety: Things To Know About Drowning
The warm weather is here and pools are open. Swimming is a great way to keep your kids cool, occupied and exercised throughout summer, however pools come with their fair share of risks. Before you take your children swimming, check out these pool safety tips. Pool safety is something every parent needs to take more seriously. Why? Because drownings of young children ages one to four have increased in recent years. Unfortunately, drownings are the number one cause of death in this age group - we lose the equivalent of 10 school buses full of children to fatal drownings in the U.S. each year. With warmer temps and hopes of cooling off in a local pool, you can’t be too careful when it comes to protecting your children from the risk of drowning. Children are naturally drawn to water, so parents must be extra aware in order to protect their kids from diving in headfirst. Kris Deeter, MD, pediatric intensive care physician at Renown Children’s Hospital, offers tips to keep your littles safe in the water. Preparing Your Child for the Pool People aren’t born knowing how to swim. This means parents must teach their children about swimming and pool safety if they want them to be safe and confident around water. It can take years to develop these skills, so the key is to start when your children are very young. Here are some ground rules: Teach your child to swim starting at age one. We recommend enrolling your toddler in swim classes; there are several organizations in the Reno-Tahoe area that offer baby and toddler swim classes. Keep your kids away from plastic and inflatable pools - they’re easy for children to fall or climb into and drown. They’re also a breeding ground for bacteria. Floaties and water wings are not safe! They are not a safe substitute or “crutch” for learning how to swim and they can lead to drowning if the child is using them incorrectly or while unsupervised. Stay within arm’s reach of babies and toddlers when at the pool. Supervision alone is not enough – you must be within arm’s reach in case they fall in and need to be rescued quickly. Learn child and infant CPR. If a drowning does occur, the best course of action is to call 911, get the child onto dry land and conduct CPR until breathing is restored or the EMTs arrive. Pool Parties: A Risk for Drowning? Surprisingly, pool parties, a common summer pastime, actually increase the risk of drowning incidents. Although responsible adults are usually at pool parties, distractions ranging from alcohol to pool toys can actually make it easier for drownings to occur unnoticed. Does this mean you should RSVP “no” to the next pool party your child is invited to? Not if you follow the pool safety tips below: Attend the party with your child so you can supervise them while they swim. Remove unused floaties and toys from the pool. They can obscure visibility, making it difficult to see a child in the pool. Don’t drink alcohol while supervising a pool party. Assign an adult “water watcher” to pay constant attention to children in the pool. Pool Safety Precautions for Homeowners If you own a pool, there are several more precautions to ensure the safety of your children. Even if your kids are strong swimmers who have mastered the rules of pool safety, there may be neighbors or friends who are younger and more vulnerable to drowning. You must undertake precautions for these children too. Some of these may seem time-consuming or expensive, but they are worth it to prevent a child from a fatal drowning. To keep your pool or spa safe, please: Cover your pool or spa when not in use. Choose a pool or spa cover with safety features like locks, safety sensors or alarms. Fence in your pool or spa area. The fence should be locked and at least four feet tall. Do not leave toys in the pool area as these may attract children.
-
Addressing the Threat of Workplace Violence in Hospitals
In recent years, workplace violence against healthcare workers has been on the rise. According to the Occupational Safety and Health Administration (OSHA), about 75 percent of nearly 25,000 reported annual workplace assaults occur in healthcare and social service settings. Those who don’t work in healthcare may be surprised to learn that violent altercations are so common in our field. Hospital settings can create fear and stress for patients and their families. Pain, mind-altering medications and drugs, and difficult prognoses can amplify these feelings. While inappropriate responses may be understandable, violence cannot be tolerated. As the leader of a health system, protecting our employees is an issue that I take seriously. Reporting Workplace Violence Unfortunately, sometimes employees don’t report dangerous incidents fearing they might be blamed, or not realizing it’s a reportable offense. At Renown Health, we take these events seriously. We have clear, mandatory policies and protocols for reporting and investigating violent incidents. Each incident is investigated to ensure follow through and accountability. We also teach de-escalation skills to our hospital security teams, clinicians, and other frontline employees. As an added layer of protection, Renown Health has a first-rate security team that closely monitors activity on our campuses, addressing potential issues before they escalate. Our organization values our partnerships with community organizations including local law enforcement agencies like the Washoe County Sheriff’s Office and the Reno Police Department. Renown Health maintains a close relationship with these partners, and we alert them when our care teams experience an increase in violent incidents. I also recognize that workplace violence is a national problem that demands collaborative solutions. That’s why I am also proud to serve as a member of the American Hospital Association’s Hospitals Against Violence Advisory Committee. Nurses, doctors, paramedics, and frontline health workers care for us every day. It’s our responsibility to support them by ensuring they feel safe at work.
Read More About Addressing the Threat of Workplace Violence in Hospitals
-
African Peanut Stew
Celebrate African Heritage and Health Week with this healthy and comforting vegan stew. Exotic spices and nutty peanuts make the flavor profile of this dish unforgettable and crave-worthy. Serve with brown rice for a complete and filling meal!
-
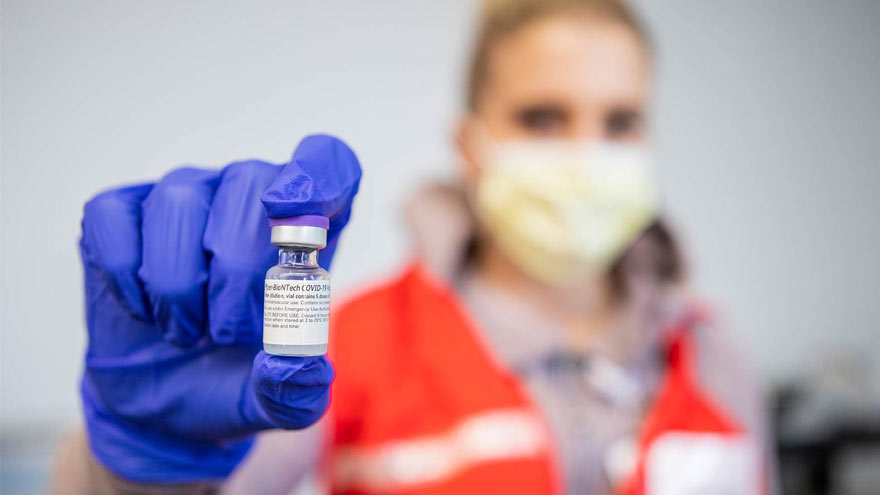
Pharmacists Answer Questions about the COVID-19 Vaccines
Vaccines that provide protection against the COVID-19 virus are bringing us closer to the end of this deadly pandemic. Two different COVID-19 vaccines are currently available in the U.S. today: one from Pfizer and the other from Moderna. Kate Ward, PharmD, BCPS, Director of Clinical Pharmacy at Renown Health and Adam Porath, PharmD, Vice President of Pharmacy at Renown, share what you need to know about these vaccines. When two COVID-19 vaccines were approved by the U.S. Food & Drug Administration (FDA) in December 2020, it was cause for celebration. Why? Because according to the CDC, the vaccines are 94 percent or more effective in providing protection against the COVID-19 virus! Many people are seeking information about the new Moderna and Pfizer vaccines. Below, our pharmacy leaders provide answers to some commonly asked questions. How do the COVID-19 Vaccines Work? The Pfizer and Moderna vaccines are both mRNA vaccines that help your immune system develop antibodies against the COVID-19 virus. The vaccines use messenger RNA, or mRNA, to show our bodies’ protein-making cells how to make the spike proteins of the COVID-19 virus. Our immune system reacts to these spike proteins by creating antibodies that can recognize and destroy them. So when a person is exposed to the virus in the future, they will be less likely to get sick. What are the Differences between the Pfizer and Moderna Vaccines? The Pfizer and Moderna COVID-19 vaccines are very similar, with just a few small differences worth noting. The main difference between the two vaccines is when you should receive your follow-up dose. Patients who receive a first dose of Pfizer should receive their second dose about three weeks later. Those who receive a first dose of Moderna should receive their follow-up vaccination roughly four weeks after their first dose. People 18 years and older can receive the Moderna vaccine while people 16 years and older can receive the Pfizer vaccine. Dosage for the Moderna vaccine is 0.5 ml (100 mcg). Dosage for the Pfizer vaccine is 0.3 ml (30 mcg).
Read More About Pharmacists Answer Questions about the COVID-19 Vaccines
-
What Does It Mean to Participate in a Clinical Trial?
Participating in a clinical trial is voluntary and a personal choice. Clinical trials are research studies that involve people and are an important part of patient care. What is a clinical trial? Clinical trials are research studies that involve people, and they are an important part of patient care. There are several different types of clinical trials; some are designed to understand trends in a disease or identify better ways to diagnose a condition, while others determine if a new treatment is safe and works when treating, improving or preventing a health condition. There are over 400,000 clinical trials currently being conducted in the United States, and even more across the world. This includes health conditions such as heart failure, cancer, Parkinson’s Disease, respiratory conditions like COPD, common infections, cystic fibrosis, and many more. Clinical trials lead the healthcare industry to new discoveries that contribute to reliable and exact care, improving healthcare quality and saving lives. Clinical trials are conducted by a team of researchers, including doctors, pharmacists and clinical research coordinators. These research teams are highly skilled in their specialty areas, often providing traditional patient care and seeing research patients in the same day. These teams are responsible for making sure the clinical trial is completed correctly, and their patients are their top priority. Why should I consider participating in a clinical trial? Participating in a clinical trial is voluntary and a personal choice. There are many reasons why patients decide to get involved in clinical research. While many clinical trials are designed for patients who have a certain health condition, many studies also ask healthy volunteers to contribute in order to compare health outcomes. Clinical trials are also for patients at all different stages of their diagnosis. Depending on the specific study, the patient may receive access to a new cutting-edge treatment before it is widely available. When patients join a clinical trial, the research team becomes a health partner dedicated to their health and well-being. When patients join a clinical trial, they make an informed decision in their healthcare by weighing all available options in addition to routine treatments. Research participants know that they are contributing meaningfully and helping other patients like them. Where can I find more information about clinical trials at Renown Health? Renown Health’s mission is to make a genuine difference in the health and well-being of the communities we serve. Renown’s clinical trial portfolio offers leading care options to patients in northern Nevada, close to home, in a variety of specialties. Contact the Renown Clinical Research Office for more information on clinical trials available to you!
Read More About What Does It Mean to Participate in a Clinical Trial?
-
Top 5 Misconceptions About Clinical Trials
There are many misconceptions about clinical research, so we have unpacked a few common myths we hear to help you make an informed decision in your healthcare. Misconception #1: If I join a clinical trial, I’ll just be a guinea pig. Quite the opposite is true! Through honest and respectful conversation, we ensure all participants are informed of the benefits and risks associated with the clinical trial during the informed consent process. Being in a clinical trial is voluntary, and we respect our patients’ decision to join or decline to participate in the clinical trial. You can always change your mind at any time as well. When patients join a clinical trial, they receive an additional team of healthcare professionals, including additional physicians, pharmacists and research coordinators, dedicated to their safety and well-being. This means that clinical trial participants often receive more support than they would in the standard treatment setting. Misconception #2: Clinical trials are too dangerous because they use new treatments that haven’t been tested. We recognize that there are different levels of risk associated with participating in a clinical trial depending on the type of study. However, new treatments are only reviewed through clinical trials after they have gone through extensive testing. New treatments that do not show promising results for safety and potential benefit during laboratory testing do not receive approval to begin clinical trials. Your research team reviews any expected benefits and risks identified from previous studies during the informed consent process, as well as any updates that occur throughout the duration of the clinical trial. The research team stays in close contact with you during the entire process, documenting and treating any side effects that you experience for both your safety and the safety of participants like you. Misconception #3: I don't want to join a trial because I could be wasting my time receiving a placebo. A placebo is a substance that has no therapeutic effect, sometimes called a “sugar pill.” Participants who receive a placebo during a clinical trial are very important, helping researchers definitively determine the specific good and bad effects of the new medication. Many clinical trials that involve a placebo also offer what is called an open label extension or cross-over study. Cross-over studies ensure that anyone taking the placebo can begin receiving the new medication, often for several years. Cross-over studies help clinician researchers understand the long-term effects of a medication while also giving patients free access to novel care for several months and even years.