Researchers at Renown Seek Convalescent Plasma Study Participants
April 20, 2021
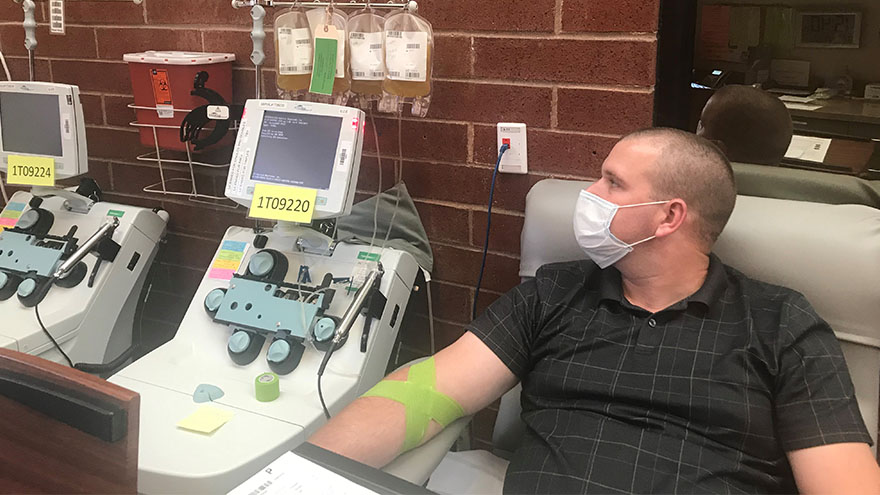
Physician researchers seek to understand how the immune system responds to COVID-19 and create a healthier Nevada.
During the early stages of the pandemic, convalescent plasma was considered the only viable treatment option available for patients with COVID-19. Convalescent plasma is the component of the blood from recovered patients that may contain COVID-19 antibodies that help fight the infection. The National Institutes of Health has since developed treatment guidelines for COVID-19 based on clinical trial data and many studies are still underway worldwide assessing various additional treatment options.
Convalescent plasma was in high demand but difficult to locate for COVID-19 patients in the northern Nevada area. A 24-year-old nursing assistant, Austin Meegan, was hospitalized and spent weeks staving off kidney and lung failure before learning he was eligible for an experimental blood transfusion that showed promise in treating COVID-19. Doctors estimated Meegan had only about a 3% chance of tracking down a donor to match his rare blood type. A COVID-19 survivor, Thomas Gibson, a Texas resident with the same blood type, traveled to Reno to donate his viral antibodies and a convalescent plasma donation credited with helping to save Meegan’s life.
Physician clinical researchers and scientists at Renown and the University of Nevada, Reno School of Medicine (UNR Med) knew they needed to create better options for patients and physicians. Clinical researchers developed a study to help other patients like Austin, and pleaded for donations from recovered COVID-19 patients to donate their convalescent plasma. The researcher teams looked to understand how the body’s immune system responds to the virus over time, to aid them in developing new treatments for COVID-19.
“The world’s capacity to get through the COVID crisis will depend on four things — science, technology, innovation and partnerships, says Tony Slonim, MD, DrPH, President and CEO of Renown Health. “Taking lab bench discoveries to the bedside of patients in an efficacious and timely manner is not easy, but with UNR Med and our partners, we are making great strides in advancing clinical research which has the power to save lives and to create a healthier Nevada.”
“It’s tremendously promising to partner on clinical research that will not only help us better understand the disease, but help inform treatment for those combatting COVID-19 that has had such a devastating impact on Nevadans, our nation and the world,” says UNR Med Dean Thomas L. Schwenk, MD.
Renown, UNR Med and other area health care partners collaborated with Vitalant to collect plasma from recovered donors for a study on the treatment's efficacy. Eligible donors are at least 18 years old, weigh more than 110 pounds and are healthy. Donors had fully recovered from a confirmed diagnosis of COVID-19.
Project coordinators at the Renown Research Office were overwhelmed by the community’s support and plasma blood donations. Additional partnerships with the Washoe County Health District, the State of Nevada and the Governor’s office, Saint Mary’s Medical Center, Northern Nevada Medical Center, Carson Tahoe Health and the VA Sierra Nevada Health Care System, along with many area health care providers helped the team meet their goals of enrolling 120 eligible participants in the study.
“Our success in this study rests heavily on the support of our great community, as well as the innovation and collaboration demonstrated by Renown and UNR Med,” said Sara Healy, MD, MPH, principal investigator of the study and a pediatric infectious disease physician at Renown Children’s Hospital and UNR Med. “We are proud to be at the forefront of conducting essential research during such a pivotal time in history, and look forward to our continued partnership as we continue this important work.”
“The control of COVID-19 in our communities relies on testing. The study that is being launched to develop a sensitive, specific and easier way to collect specimens (blood) is advancing the field and brings promise towards getting to our common goal of having the right diagnostic test for the right clinical situation at the right time,” says Mark Riddle, MD, DrPH, FISTM, associate investigator of the study and associate dean for clinical research and professor at UNR Med's Department of Internal Medicine and Medical Research.
The research team is now asking area residents to participate in a study to analyze the efficacy of two COVID-19 tests. Participants will undergo two blood tests: one being a finger stick to provide results for a rapid test, and the other is a traditional venipuncture draw confirming the presence or absence of COVID-19 antibodies. This study is a collaboration with InBios International, Inc., a leading biotechnology company based in Seattle.
Researchers are seeking:
- Individuals who have confirmed positive for COVID-19 and who have recently recovered from the virus. Study participants must be within 7-28 days from the onset of their symptoms.
- Individuals who have recently tested negative for COVID-19 and have never tested positive.
Those who are interested in participating in the study may contact project coordinators at the Renown Research Office at (775) 982-3646, or via e-mail at covidplasmascreening@renown.org, 7:30 A.M. – 5:00 P.M., Monday through Friday.
Individuals aged 18-75 in general good health are encouraged to consider participating in this ongoing study. There is no cost to participate in this study and participation is voluntary. An individual’s decision to participate will not affect their current or future relations with their health care provider(s), health district, or the community. Those who decide to participate are free to withdraw at any time.
“Time is of the essence with COVID. If we can get test results to people and their clinicians in a more timely way, we can make a faster diagnosis of a patient's condition, says Christopher M. Kozlowski, MD, MHA, Renown's institutional research officer and Medical Director/VP of Renown Institute for Heart & Vascular Health. “As we refine the accuracy of our testing; we are applying sensitivity and specificity testing for true negative and true positive results. This provides people with more timely and accurate results and better quality care.”
About Renown Health
Renown Health is a locally governed, not-for-profit integrated healthcare network serving northern Nevada, Lake Tahoe and northeast California. Renown is one of the region’s largest private employers with a workforce of more than 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown has a long tradition and commitment to improve the care and the health of our community.
About the University of Nevada, Reno School of Medicine
The University of Nevada, Reno School of Medicine (UNR Med), Nevada’s first medical school, is a community-based, research-intensive medical school with a statewide vision for a healthy Nevada. Established in 1969, UNR Med is improving the health and well-being of all Nevadans and their communities through excellence in student education, postgraduate training and clinical care, research with local, national and global impact and a culture of diversity and inclusion. For more information, visit med.unr.edu.